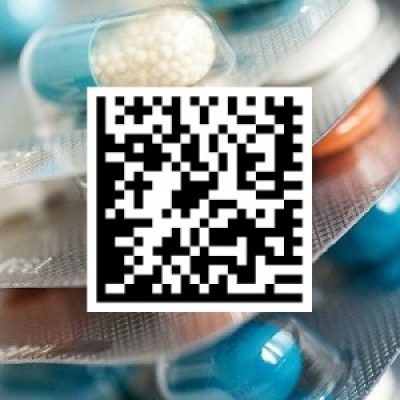
Starting March 1, all participants must be registered in the state drug movement monitoring (DMM) system. It is assumed that their information systems are already on alert for interaction with the state information system "DMM". To date, the most prepared link in the entire distribution chain are pharmaceutical manufacturers. But in the industry, there are still representatives of pharmacies and medical institutions who ask: what is labeling? Meanwhile, 116 days are left before the start. July 1, 2020 the law comes into force.
The experiment goes to the finish line. After 4 months, the system should be fully operational, however, unresolved issues remain. The Director General of the Association of Russian Pharmaceutical Manufacturers Viсtor Dmitriev notes that it is necessary to ensure legal support of the process at all levels within the framework of by-laws regulation. “In the course of introducing the labeling, we see that there are remaining zones of legal voids. It is not clear how reverse logistics will take place if, for some reason, the drug is rejected? In addition, experience shows that today the system does not keep pace with the novelties in the legislation. So, in our regulatory framework, a manufacturer can transfer goods to a distributor for storage, but these business processes are not registered in DMM. And there is no solution yet,” said the head of the ARPM.
According to the executive director of the “Inpharma” Association, Vadim Kukava, it is premature to talk about the system’s readiness. “Since July 1 of this year, the system should work, the working system is working all the elements, not only manufacturers, distributors, but also a pharmacy somewhere in Vladivostok, it should also work. How this system will work is not clear,” said Kukava.
According to analysts, the reasons for the unpreparedness should be sought in the perception of the situation by the business, which perceives the labeling as a kind of fiscal system, from which there is and will not be any benefit. Unfortunately, the issue of providing free data on the movement of goods throughout the chain has not yet been resolved. This information is extremely important for manufacturers, it will replace expensive marketing research and will allow planning the workload. In addition, constant changes to the rules during the experiment also reduced the degree of trust in the project.
So the industry again raises the question of reducing code length. This will not only significantly reduce the cost of the final product, but also reduce the amount of defects when applying the code to the package. According to experts, from July 1, an average price increase of 2-2.5 rubles per package is expected. “It will not be so noticeable on expensive drugs, but on the low price segment it will be noticeable,” Victor Dmitriev noted.
Will there be another rule change? Will the code fee be reduced? And is it worth waiting for the deadline postponing? “In May, deputies of the State Duma together with Federal Service for Surveillance in Healthcare (Roszdravnadzor) will check the readiness of the system along the entire chain, only after a detailed analysis will it be decided to launch the system,” Victor Dmitriev answered.
In the meantime, manufacturers are the most prepared link in the entire chain. Entire project teams are working on the introduction of labeling. Big questions to the work of pharmacies and hospitals. To date, outlets are being created — stocks for the uninterrupted supply of drugs without labeling for patients.
ARPM Press Service